“The presence of highly pathogenic microbes can present high-risk mutations and can be fatal to patient. Their presence needs to be urgently and efficiently remediated.”
Microbial contamination is a risk to product quality and safety.
Microbial contamination has a huge impact on product quality as they introduce variability and can cause loss of potency due to degradation and modification of the product by microbial enzymes, changes in the impurity profiles, and an increase in the level of bacterial endotoxins.
Examples of FDA Warning Letter for common problems found in aseptic operations:
- “Your firm failed to establish and follow appropriate written procedures that are designed to prevent microbiological contamination of drug products purporting to be sterile, and that include validation of all aseptic and sterilization processes (21 CFR 211.113(b).“
- “The company’s investigation into the media fill flub was “insufficient,” the FDA said. “Without a comprehensive assessment of contamination hazards and a [Corrective Action and Preventative Action (CAPA) plan] that builds more holistic risk mitigation into your operational design, there is no assurance that you can prevent recurrence of sterility problems due to various latent or active failure modes in your operation.”
- “Lack of controls to prevent contamination of drug products. FDA identified conditions at facilities that pose a significant microbial contamination risk. For example, poor facility maintenance, including leaking pipes in the cleaning room ceiling, chipped, and cracked floors in the batch tank room, and blue and black particulates as well as dust on tanks next to the ingredient charging ports
- Failure to thoroughly investigate contamination, and releasing lots without meaningful scientific justification. (A negative retest, alone, is NOT sufficient)”.
- “ FDA noted that the firm identified microbial contamination with a positive test result, yet distributed the lots that tested positive after a “clean” retest without a scientific basis to invalidate positive test results
- No evidence of lab error”.
- “Failure to identify a root cause of the contamination and setting forth a scientific rationale to discount the firm’s production operation was not the source of the microbial contamination”.
Inspection Trends:
Inspection trends and common problems investigators are seeing in auditing manufacturing facilities are related:
– Media fill operations,
– Sterility testing, and
– Environmental monitoring.
What do you need to do?
Poor root cause investigations will not arrive at the root cause of product contamination. The company needs to perform a comprehensive CAPA root cause analysis and risk assessment in order to identify the root cause , identify adequate corrective actions and areas for improvements to prevent recurrence of failures .
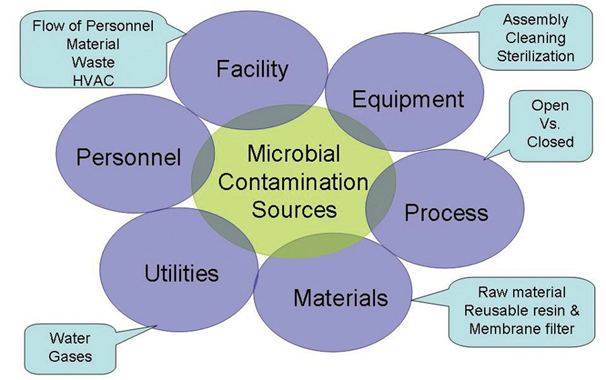
The biocontamination hazard assessment: A hazard analysis (biocontamination hazard analysis) and critical control points assessment for bioburden throughout the manufacturing process is useful for the design of a microbial control strategy and the performance of a systematic investigation. Also, it is important to track the failure data to gain a better understanding of the root causes. The information should be used to continuously evaluate risks and implement process/and or equipment improvements to mitigate and prevent microbial contaminations.
In general, the design of the facilities should allow for proper operations and prevention of contamination. The flow of the personnel, material and waste should be from clean to dirty areas and critical upstream, open operations liable to microbial contamination should be performed in designated biosafety hoods or areas with ISO 5 classification. Segregation of areas, appropriate changeover SOPs should be in place. Environmental monitoring of the manufacturing areas should be performed at appropriate intervals. Process gases and water should be tested and monitored to ensure adequate microbial control. The design of the equipment (single use disposable versus multi use, validated cleaning and sterilization process along with a comprehensive preventive maintenance plan are critical components of the microbial control strategy.
It is important to identify and establish processing steps that decrease the bioburden and bacterial endotoxin levels. Bioburden and endotoxin alert and action limits should be set for process steps based on process capability.
Raw materials should be screened for microbial quality and should be stored in a manner to prevent contamination and cross- contamination. Personnel performing operations should be trained adequately and evaluated periodically in such operations.


