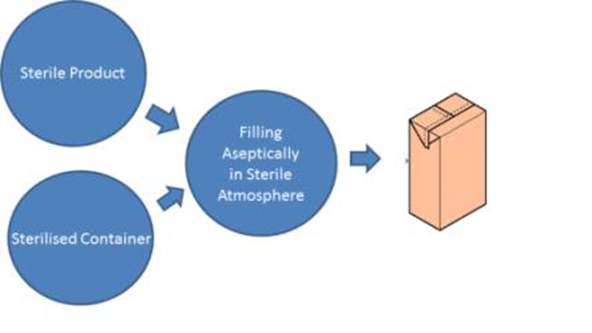
Aseptic Processing:
“Aseptic processing is defined as “handling of sterile products, containers, and/or devices in a controlled environment, in which the air supply, materials, equipment, and personnel are regulated to maintain sterility”
(ISO 13408-1, 2008).
Aseptic processing is a manufacturing method that can produce product that is absent of bacteria without subjecting the product to terminal sterilization processes. Many products degrade and become ineffective when subjected to the harsh conditions of terminal sterilization. Aseptic process manufacturing allows these products to be produced in a sterile environment, allowing them to maintain their effectiveness while being safe to inject into patients.
Aseptic Techniques:
Aseptic technique is a method that involves target-specific practices and procedures under suitably controlled conditions to reduce contamination from microbes.
Examples of Aseptic Techniques:

- Hand hygiene is important for preventing infection transmission ..
- Barriers Sterile gloves, gowns, masks, and drapes should be used. …
- Decontamination techniques …
- Environmental control …
- Clean room, clean room control, and testing…
- Controlled Manufacturing Environment …
- Utilities.. water, compressed air…..
- Isolators……
- Laminar Flow…
- Temperature, humidity
- Air pressure……
What is bioburden and how can it be controlled:
Bioburden is the number of contaminated microorganisms found in a given amount of material before a sterilization procedure is carried out. Bioburden levels are measured in terms of colony-forming units (CFUs). These units are an estimation of the number of viable bacteria or fungal cells found on a product sample.
When your medical device is being assembled or manufactured, you want it to have the lowest possible level of bioburden, because the lower the bioburden level, the easier it is to sterilize your product.
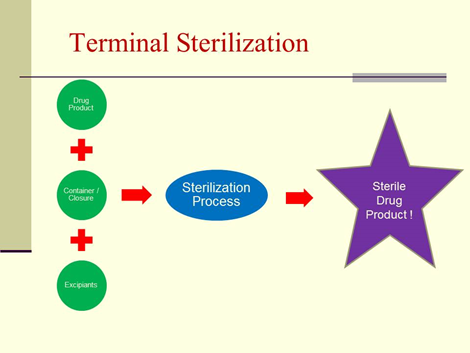
Terminal Sterilization:
Terminal sterilization is the process of sterilizing products in their final container.
Terminal sterilization is achieved by exposure to a physical (e.g., temperature, radiation) or chemical sterilizing agent (e.g., Vaporized Hydrogen Peroxide (VHP), Vaporized Peracetic Acid (VPA), Ethylene Oxide (EO) for a predetermined extent of treatment. It provides a SAL (Sterility Assurance Level) that is possible to calculate, validate, and control, and thus incorporates a safety margin.
Terminal sterilization ensures that no viable microbial contaminants like fungi or bacteria are present when the product is used.
“The presence of highly pathogenic microbes can present high-risk mutations and can be fatal to patients. Their presence needs to be urgently and efficiently remediated.” FDA’s Brooke Higgins.